Clinical Trials for Everyone.
Olympia Clinical Trials
Our focus is patient safety and innovative research for the benefit of public health
Trials
What are clinical trials?
Clinical research is a branch of healthcare science that determines the safety and effectiveness of medications, devices, diagnostic products and treatment regimens intended for human use.
All information we collect will be strictly confidential as per the Health Insurance Portability and Accountability Act of 1996 (HIPAA). The information we collect about you will be de-identified. In addition you may be compensated for your participation in a trial, depending on the trial specifications.
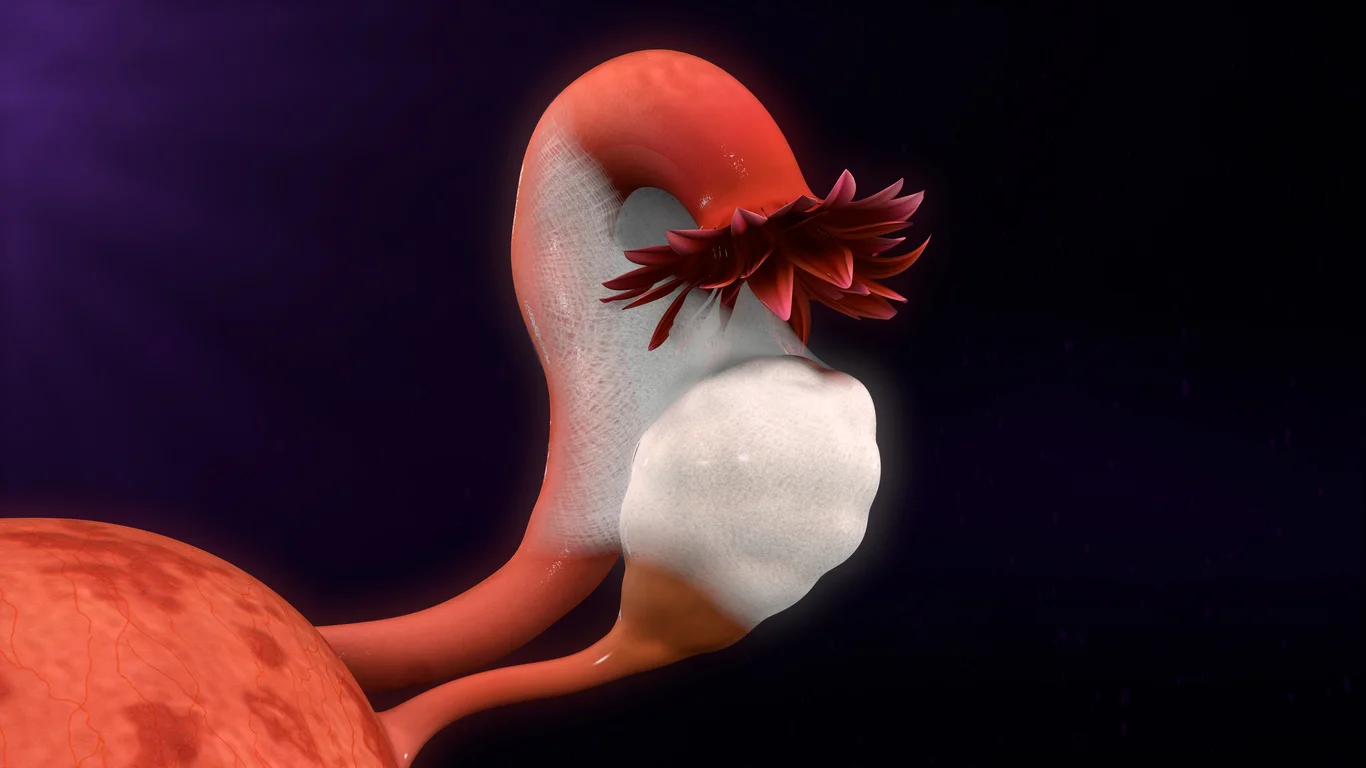
The objective of this study is to evaluate safety and efficacy of elagolix in the management of moderate to severe endometriosis-associated pain in adult premenopausal female subjects including the safety and efficacy of elagolix in combination with concomitant hormonal add-back therapy.

Do heavy periods assocaited with uterine fibroids affect you? Uterine fibroids symptoms can affect woman, anytime, anywhere. If you're at least 20 years old and have heavy periods associated uterine fibroids, you may qualify for this oral investigational medication research study. As a participant, you will receive all study-related care.
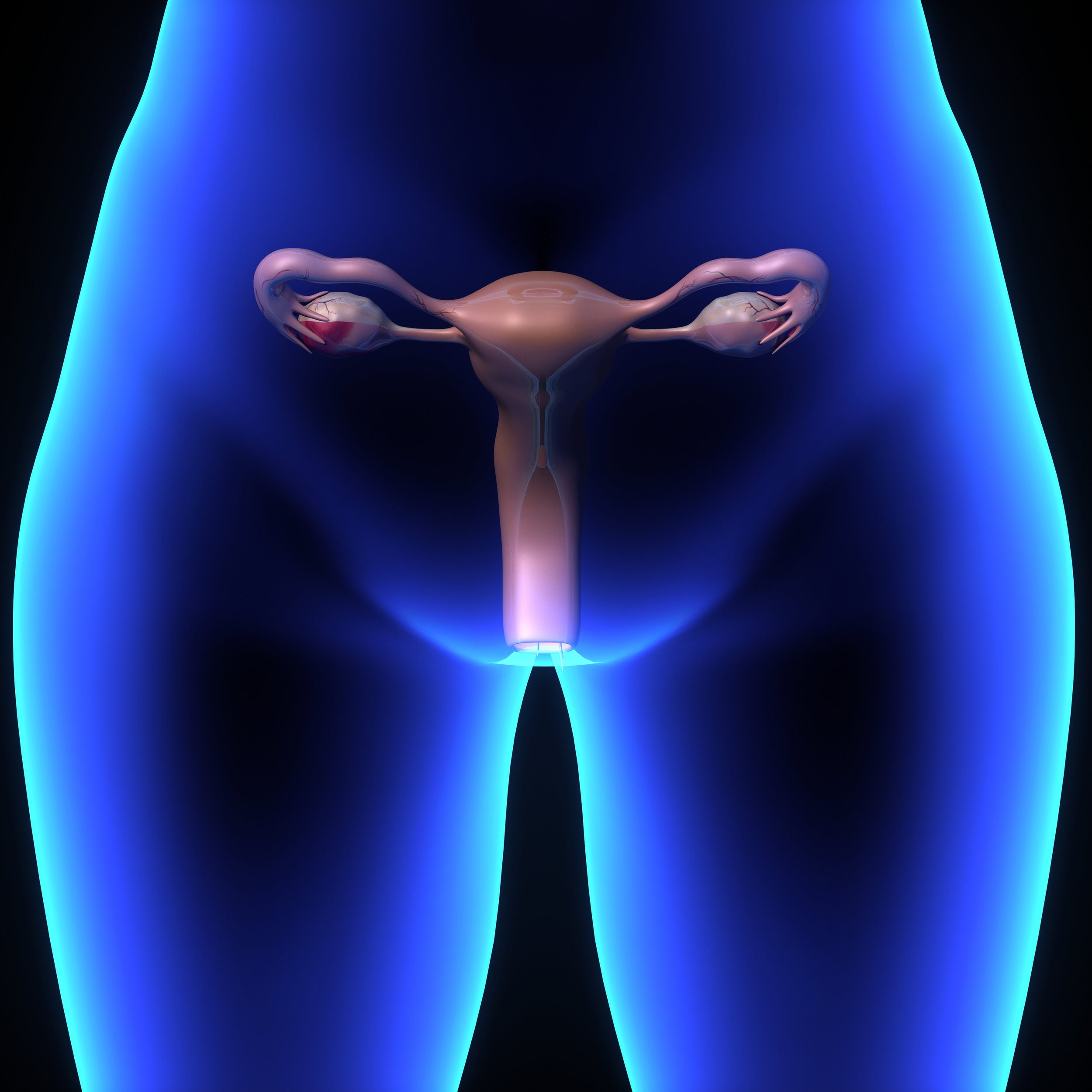
If you are interested in additional trials, please subscribe for updates below.
Participate
Why should you participate?
By enrolling in a clinical trial, you can contribute to society by helping to develop new drugs, therapies and medical devices. You can be a vital part of a ground-breaking scientific discovery. In addition, you may recieve compensation for your time and effort, depending on the trial specifications. Being part of a clinical trial allows you access to the most cutting-edge treatments available for you.
Research
Who can participate?
Elegibility or inclusion criteria are the characteristics required to participate in a clinical trial. Your eligibility may be based on age, gender, overall health, type and stage of disease, treatment history, and other conditions.
Each clinical trial targets a specific patient population so inclusion and exclusion critera must be stringently followed.